- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Sinae APIs Categoria Factory
- View as
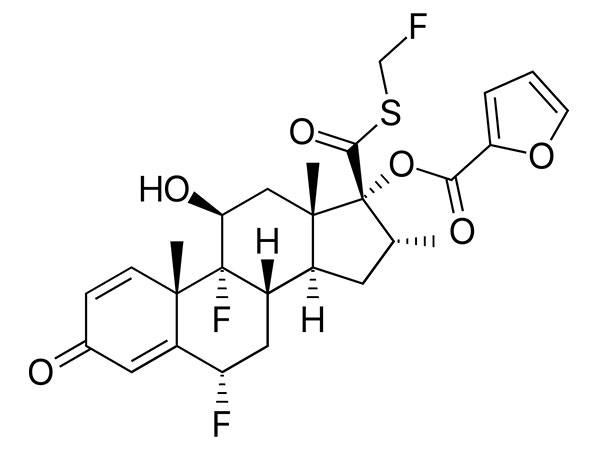
Fluticasone Furoate
Fluticasone furoate has in-domus specificationes. DMF fub interpositione.
CAS:397864-44-7
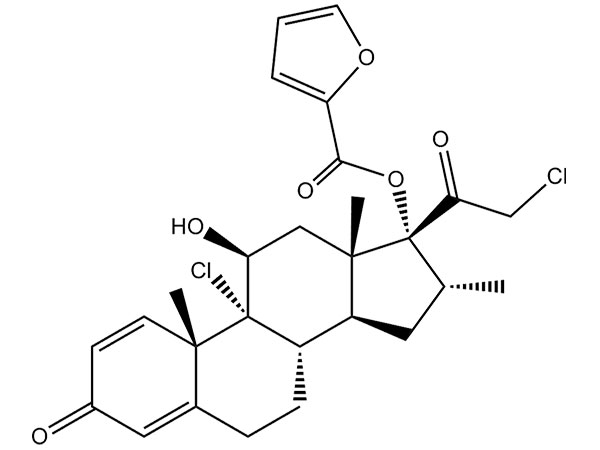
Lege plusMitte InquisitionemMometasone Furoate
Mometasone furoate speciem habet CPãEPãUSP. DMF fub interpositione.
CAS:83919-23-7
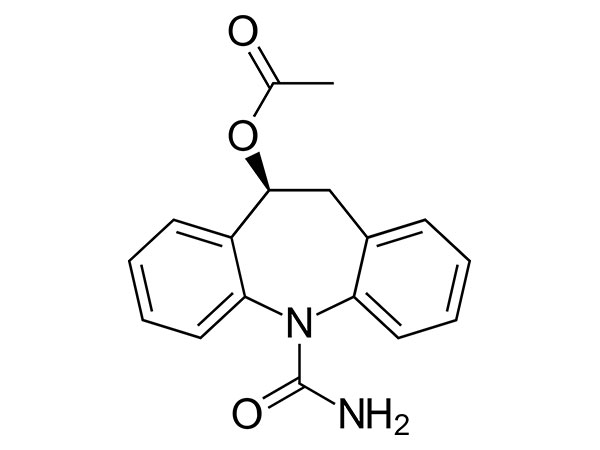
Lege plusMitte InquisitionemEslicarbazepine Acetate
Acetate Eslicarbazepine habet specificationem In-domus. DMF probatus.
CAS:236395-14-5
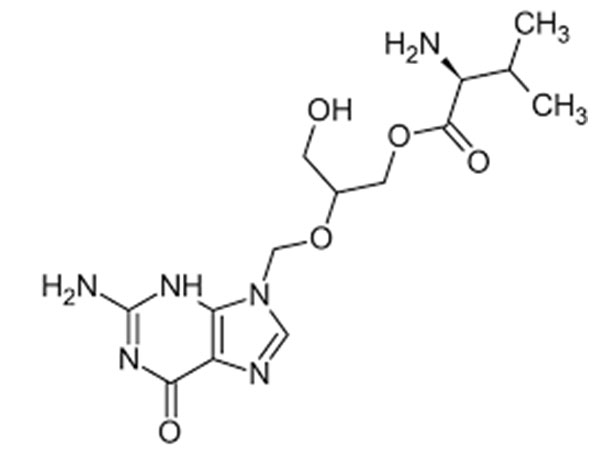
Lege plusMitte InquisitionemValganciclovir hydrochloride
Valganciclovir hydrochloride USP specificationem habet. DMF probatus.
CAS:175865-59-5
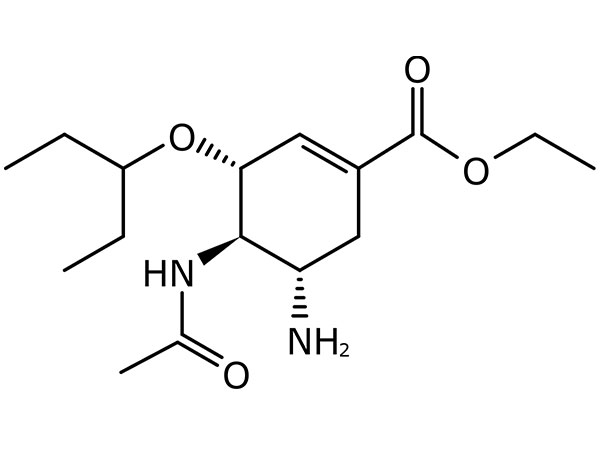
Lege plusMitte InquisitionemOseltamivir Phosphate
Oseltamivir Phosphate CP, EP et USP specifications.DMF in promptu habet.
CAS:204255-11-8
Lege plusMitte InquisitionemRevefenacin
Revefenacin habet specificationem in domo. DMF probatus.
CAS:864750-70-9
Lege plusMitte Inquisitionem
Humanwell Pharmaceuticus unus ex maximis API fabricandis in Sinis. Cum plusquam XX annos experientiae, APIs, intermediis et formulis elaboramus, fabricamus et mercaturas steroidales. Forum nostrum toto orbe terrarum operit, praesentias validas habemus in America Septentrionali, Europa, America Meridionali et Africa, cum fructibus plus quam 150 terris venditis.