- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Sinae APIs Categoria Factory
- View as
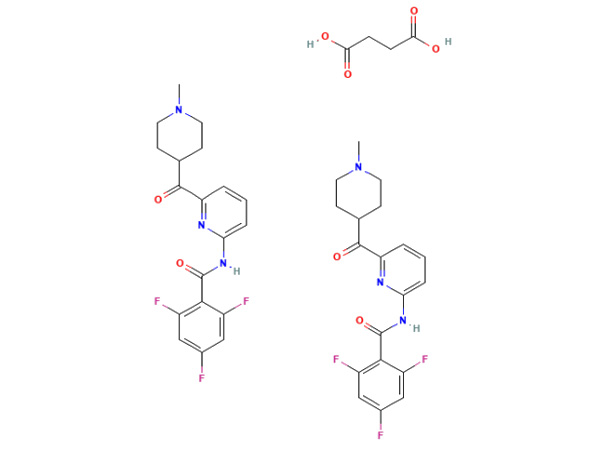
Lasmiditan Succinate
Lasmiditan Succinatus habet specificationem In-domus. DMF probatus.
CAS:439239-92-6
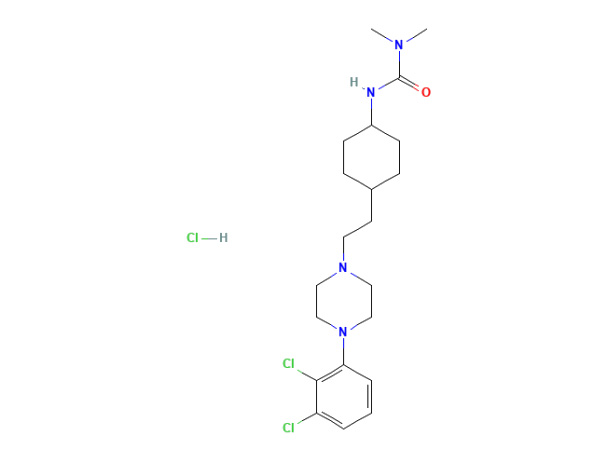
Lege plusMitte InquisitionemCariprazinum hydrochloridum
Cariprazinum hydrochloridum in specificatione-domum habet. DMF probatus.
CAS: 1083076-69-0
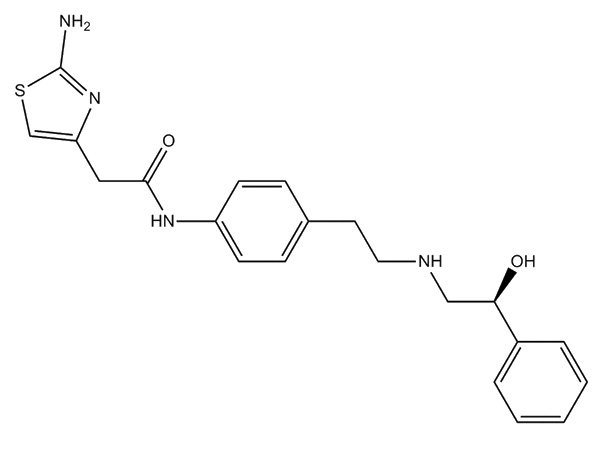
Lege plusMitte InquisitionemMirabegron
Mirabegron habet specificationem in domo. DMF probatus est.,.
CAS:223673-61-8
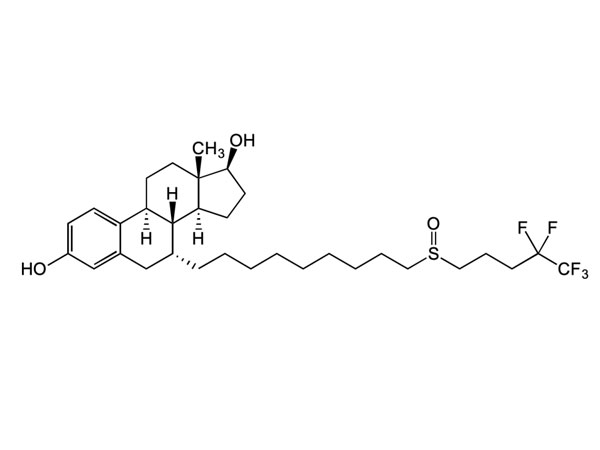
Lege plusMitte InquisitionemLuliconazole
Luliconzole habet in-Domus specificationem: DMF probatus.
CAS: 187164-19-8
Lege plusMitte Inquisitionem
Humanwell Pharmaceuticus unus ex maximis API fabricandis in Sinis. Cum plusquam XX annos experientiae, APIs, intermediis et formulis elaboramus, fabricamus et mercaturas steroidales. Forum nostrum toto orbe terrarum operit, praesentias validas habemus in America Septentrionali, Europa, America Meridionali et Africa, cum fructibus plus quam 150 terris venditis.